/ Magazine / Other Articles / From chemistry to sustainability: the role of detergents in water washing
by ING. VITTORIO CIANCI
Director LART – Textile Analysis and Research Laboratory
In water-based washing, the selection and proper use of detergents are essential for effective soil removal, fibre protection, and high-quality garment care. Detergents can be applied at various stages of the process: during washing as standard detergents, before washing as pre-spotting agents, or after washing as stain removers. Their performance depends on chemical composition, the type of soil, and the specific operating conditions.

Detergents are generally classified as natural or synthetic. Natural detergents consist of traditional soaps made from alkaline salts of fatty acids, such as palmitic and stearic acids. A common example is neutral soap, frequently used for delicate fabrics like wool. This type of soap is produced by fully neutralizing free fatty acids and removing excess lye to achieve a pH close to neutral. In practice, a perfectly neutral soap would have little to no cleaning power, so “neutral” soaps retain a slightly alkaline pH to ensure effective washing.
However, soaps are not well suited for domestic machine washing. They are sensitive to water hardness and react with calcium salts, forming insoluble deposits on fabrics that can leave them dull, stiff, and streaked. For this reason, domestic laundry relies almost exclusively on synthetic detergents. Derived from petroleum products, these detergents are specifically formulated to perform effectively in hard water. Their composition typically includes surfactants, alkalis, chelating agents, anti-redepositing agents, chemical bleaches, enzymes, and other functional additives that enhance cleaning performance.

Surfactants are the key molecules responsible for tackling grease and dirt. They have a unique dual structure: one end is attracted to water (hydrophilic), while the other clings to oils and fats (lipophilic). This allows them to lift dirt away from fabrics, ensuring clothes come out clean and fresh.
Grease and oil stick stubbornly to fabric fibers, and water alone can’t remove them. That’s where surfactants come in. Their dual nature lets one end attach to the grease (lipophilic end) while the other connects to water (hydrophilic end), forming a bridge that lifts dirt away. The result: stable emulsions that carry the grease off the fabric, allowing it to be rinsed away during washing. One of the key effects of surfactants is reducing surface tension. Stains that initially spread flat across fibers contract into a more rounded shape, increasing the contact angle and making them much easier to lift and remove from the fabric.
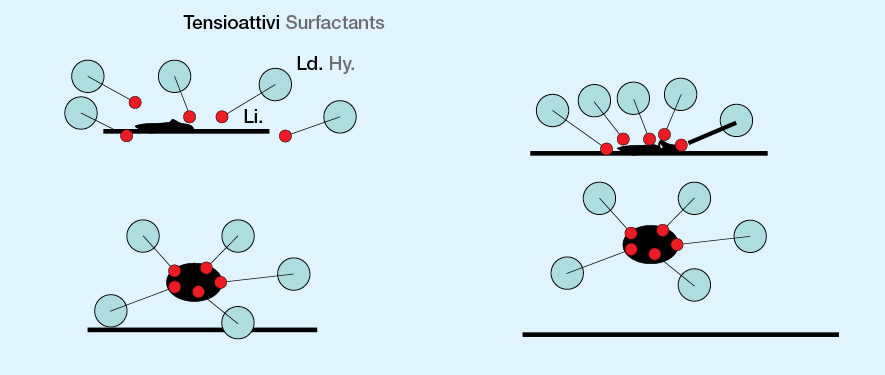
The key to effective soil removal lies in surfactant concentration: the more surfactant present, the easier it is to lift dirt from fabrics. Agitation, warm water, and repeated rinses further boost cleaning power, ensuring even stubborn stains are thoroughly removed.

Alkalis are mineral substances with an alkaline reaction that boost the effectiveness of surfactants.
Their main functions include:
• reducing the surface tension of water
• allowing fabrics to wet more easily
• neutralizing acidic soils
• saponifying greasy deposits
• dispersing solid particles
Some of the most commonly used alkalis include caustic soda, sodium carbonate, and calcium hydroxide. However, they are sensitive to water hardness and can be harsh on fibers, especially at high temperatures
Detergents also include a variety of auxiliary products designed to enhance cleaning and protect fabrics:
• Chelating agents: they bind calcium and magnesium salts that cause water hardness, as well as metals like iron and copper that can damage fibers. A common example is sodium tripolyphosphate.
• Anti-redepositing agents: keep soils suspended in the wash water to prevent them from settling back onto fabrics.
• Optical brighteners: enhance the whiteness of fabrics by converting UV light into visible light, giving a brighter, more vibrant appearance.
• Enzymes: proteins that accelerate the breakdown of proteinbased, fatty, and starchy stains. Advances in bioengineering have made enzymes more stable and highly specific to different types of soils.
• Neutralizers: convert residual alkalinity into neutral salts to prevent yellowing and neutralize active chlorine, protecting fabrics from chemical damage. Common examples include acetic acid, formic acid, bisulfite, and hyposulfite.
Water hardness is a key factor affecting laundry performance. In Italy, it is measured in French degrees (°dF), where 1 °dF corresponds to 1 gram of calcium carbonate in 100 liters of water.

Soft water, typically found in mountainous regions, is ideal for soaking, pre-washing, and washing phases, as it enhances detergent effectiveness. Hard water, more common in coastal areas, can be beneficial during final rinses, helping remove residual detergents from fabrics. Water hardness can be effectively managed through water-softening systems or by adding phosphates and polyphosphates during the washing process.
Bleaching is used to remove natural discoloration and stains caused by soils.
It can be carried out using two main approaches:
• oxidizing bleaching agents (hypochlorite, perborate): effective but potentially harmful to fibers
• reducing bleaching agents (hydrosulfites, sulfites): gentler but less stable over time. Optical bleaching, in contrast, does not remove stains but masks yellowing using fluorescent dyes. These dyes enhance reflectance in the violet-blue spectrum, making fabrics appear whiter and brighter.
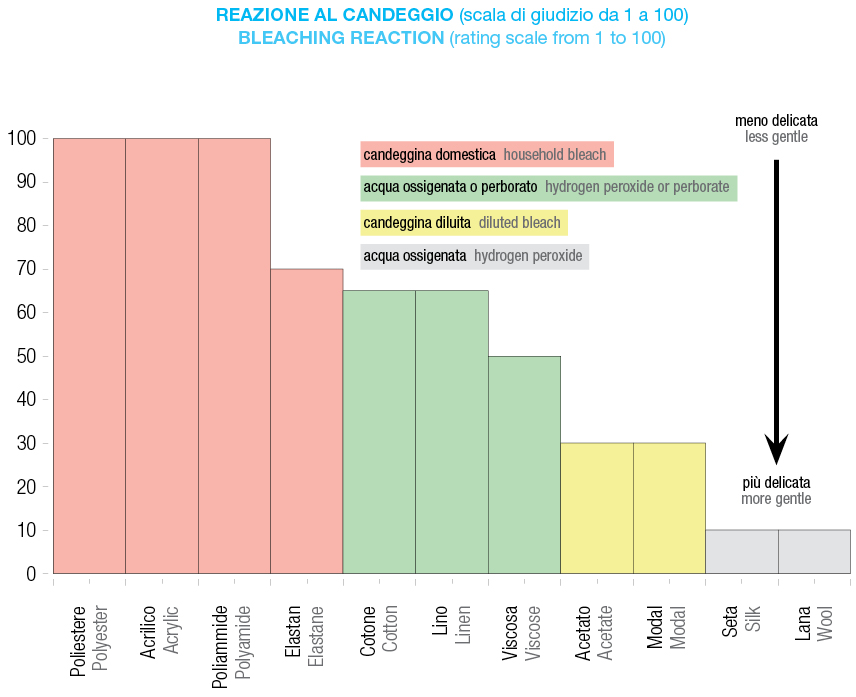
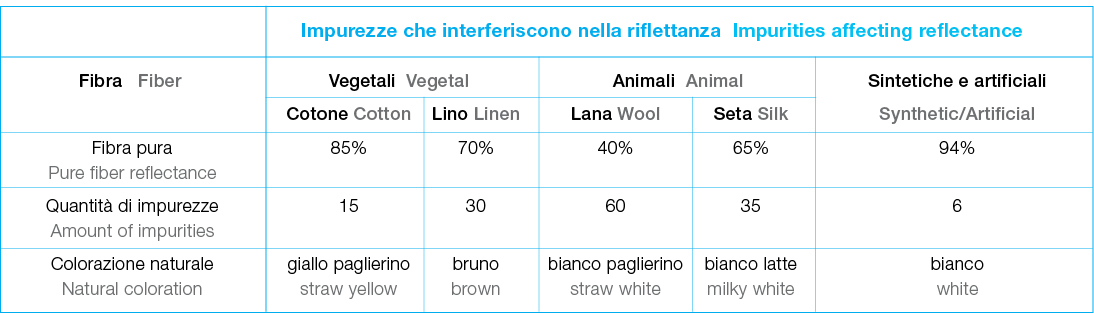
Optical bleaching, in contrast, does not remove stains but masks yellowing using fluorescent dyes. These dyes enhance reflectance in the violet-blue spectrum, making fabrics appear whiter and brighter.
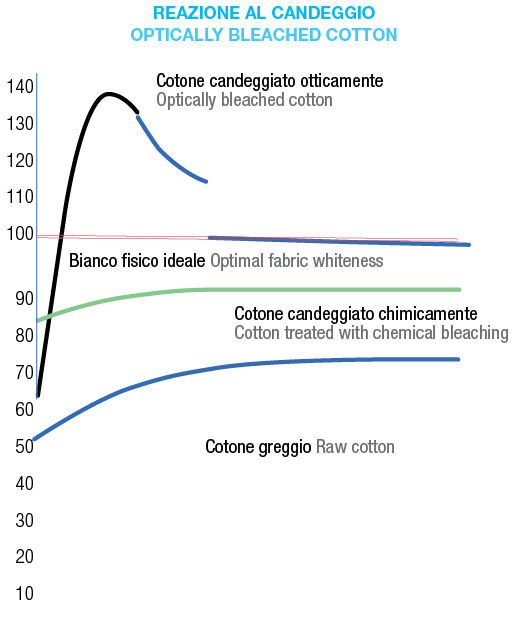
Water-based washing remains the most effective method for removing stains and ensuring proper sanitization.
However, it also raises environmental concerns:
• wastewater contains surfactants, enzymes, softeners, and phosphates
• some of these substances can be toxic to aquatic ecosystems
• phosphates contribute to eutrophication, promoting excessive algae growth in water bodies
• microplastics are released during washing, approximately 37,84 mg/kg by hand and 222,84 mg/kg in a washing machine • water consumption is high, averaging around 45 liters per domestic cycle.
Hydrocarbon cleaning is performed in a closed-circuit system, preventing direct discharge into the environment. Waste production is minimal: about 5 kg of waste for every six 30 kg washes, containing only 2,3 g of solvent per kg of waste. This method avoids the release of microplastics, and the solvents used are relatively mild compared with alkaline detergents. •
Water washing delivers the most efficient dirt removal and garment sanitization. In contrast, hydrocarbon cleaning provides an eco-friendlier alternative, thanks to its closedcircuit system and gentler impact on fibers. Choosing the most suitable method depends on factors such as fabric type, soil characteristics, and sustainability goals.
DETERGO MAGAZINE # JANUARY 2026
Share
Do you want to become one of us?
Do you know the association?
In 1990 was founded the Association ASSOFORNITORI. In 2022 the name has been changed to ASSOCIAZIONE ITALIANA FORNITORI LAVANDERIE (ITALIAN ASSOCIATION OF LAUNDRY SUPPLIERS), with the acronym AIFL.
Become a Member
Phone: 02 39 31 41 20
Email: info@assofornitori.com
C.F. 97091250155
Via Aldo Moro 45
20060 Gessate (MI)
detergo
Associated Companies
Phone: 02 39 31 41 20
Email: info@assofornitori.com
C.F. 97091250155
Via Aldo Moro 45
20060 Gessate (MI)
Follow us